When a drug can save your life but also seriously harm you, how do you make sure it’s used safely? That’s the exact problem the FDA solved with REMS programs - Risk Evaluation and Mitigation Strategies. These aren’t just paperwork. They’re legally required safety nets for medications with dangerous side effects, like birth defects, organ failure, or life-threatening blood disorders. Without REMS, drugs like thalidomide, clozapine, or lenalidomide might never have reached patients who desperately need them. But these programs don’t come without cost - to patients, doctors, pharmacists, and the system as a whole.
What Exactly Is a REMS Program?
A REMS program is a formal plan the U.S. Food and Drug Administration (FDA) requires for certain prescription drugs that carry serious safety risks. It’s not optional. It’s not a suggestion. If the FDA determines that a drug’s benefits outweigh its risks - but only if specific safeguards are in place - then the manufacturer must build and run a REMS program. This started in 2007 under the Food and Drug Administration Amendments Act (FDAAA), giving the FDA clear legal power to enforce these programs.
Before REMS, the FDA relied on patchwork systems. For example, isotretinoin (Accutane) had a voluntary pregnancy prevention program in the 1980s. Thalidomide had strict controls after its link to severe birth defects. But those systems weren’t consistent. REMS brought standardization. Today, about 120 REMS programs are active, covering roughly 185 drugs - that’s over 5% of all prescription medications in the U.S.
The Three Core Parts of Every REMS
Not all REMS are the same, but every program includes at least one of three components. The most common is the Medication Guide. These are printed handouts given to patients that explain the drug’s risks in plain language. About 78% of REMS programs require them. Think of them like warning labels you can actually read.
The second part is the Communication Plan. This targets doctors and pharmacists. It can include letters, training materials, or safety alerts. About 62% of REMS programs use these to make sure prescribers understand the risks and how to manage them. For example, a doctor prescribing clozapine - a powerful antipsychotic that can wipe out white blood cells - must be trained on how to monitor blood counts.
The most restrictive part is the Elements to Assure Safe Use (ETASU). This is where things get serious. About 45% of REMS programs include ETASU. These are the heavy-duty controls. They might require:
- Doctors to get certified before prescribing
- Pharmacies to be specially licensed to dispense the drug
- Patients to enroll in a registry
- Monthly blood tests or pregnancy tests
- Only allowing the drug to be given in hospitals or specialty clinics
Take lenalidomide (Revlimid), a leukemia drug. Its REMS requires every prescriber to be certified, every patient to be enrolled, and women of childbearing age must take two pregnancy tests before each refill. If you’re a pharmacist, you can’t fill the script unless you check all these boxes in the online REMS portal.
How REMS Gets Approved - And Why It Takes So Long
When a drug is being reviewed for approval, the FDA decides whether a REMS is needed. Sometimes it’s clear from the start - like with thalidomide, where the risk of birth defects was well known. Other times, a REMS gets added years later after new safety data comes in.
Once the FDA says a REMS is required, the drug company has 120 days to submit a detailed plan. That plan must show how they’ll meet safety goals, who’s responsible for what, and how they’ll measure success. The FDA doesn’t just rubber-stamp it. They evaluate it using a benefit-risk framework: How bad is the risk? Are there alternatives? Will this REMS actually help - or just slow things down?
After approval, the manufacturer must keep submitting reports. Every year, they have to prove the REMS is working. If it’s not helping, the FDA can remove it. Only three REMS have been discontinued since 2007. The most recent was for Zeposia, a multiple sclerosis drug, in March 2023 - after the FDA found the risks were well managed through regular labeling.
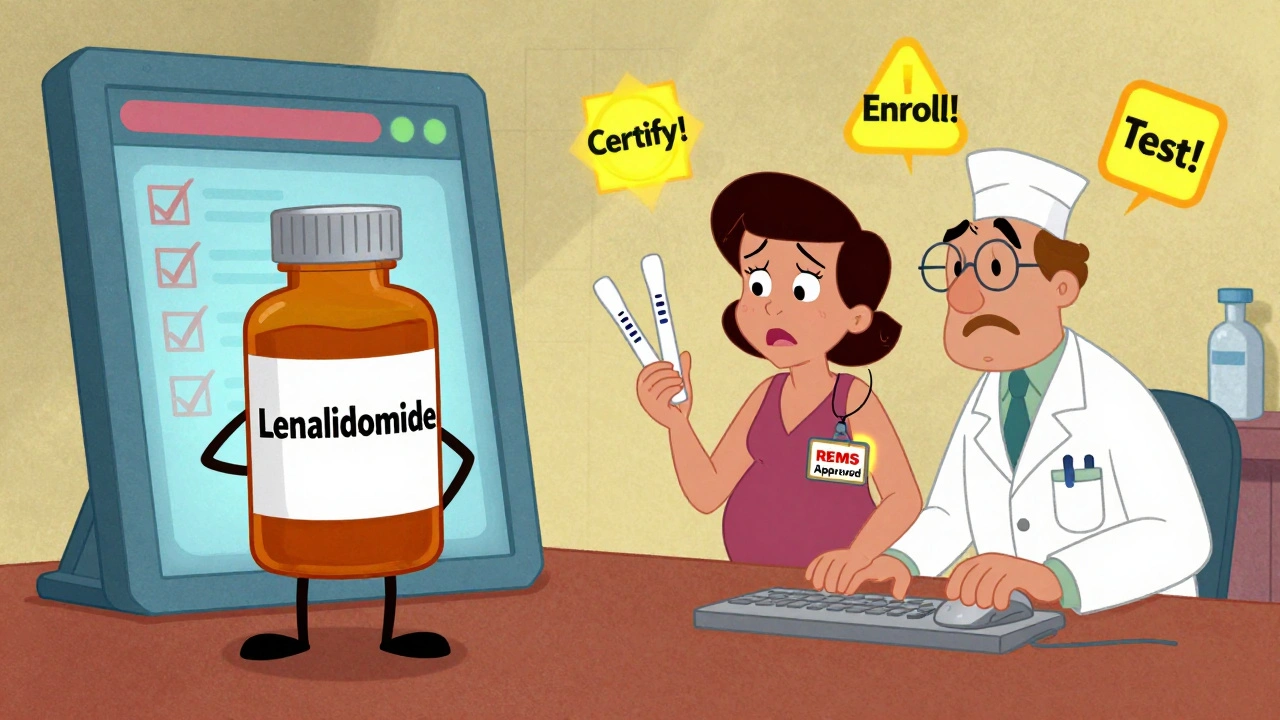
Who Pays the Price - And Who Bears the Burden?
REMS programs cost money. A lot of it. The FDA estimates each REMS program costs manufacturers an average of $2.7 million per year. That’s for building portals, training staff, printing materials, and running registries. But the cost doesn’t stop there.
Doctors spend time. A 2024 survey by the American Medical Association found that completing certification for one REMS program takes about 45 minutes. For a hematologist managing five different REMS drugs? That’s nearly four hours a week - just to stay compliant. A 2023 survey by the American Society of Hematology found 68% of hematologists spend more than five hours a week on REMS paperwork alone.
Pharmacists are caught in the middle. For drugs like Entyvio or Lemtrada, they must log into online systems, verify prescriber certification, check patient enrollment, and document everything. One pharmacist on Reddit said Entyvio REMS adds 15 to 20 minutes per prescription just for verification. That’s time taken away from other patients.
Patient delays are real. A GoodRx survey in 2023 found 42% of patients prescribed a REMS drug experienced at least one treatment delay. Sometimes it’s because the doctor hasn’t been certified. Sometimes the pharmacy doesn’t carry the drug. Sometimes the patient’s pregnancy test result hasn’t come back yet. These aren’t minor hiccups - they’re life-altering delays for people with cancer, MS, or autoimmune diseases.
Why REMS Blocks Generic Drugs
One of the biggest criticisms of REMS is how it slows down generic competition. In theory, REMS should be the same for brand and generic versions of the same drug. In practice, it’s not that simple.
Generic manufacturers need samples of the brand-name drug to prove their version works the same. But if the brand company controls the REMS - and the only way to get samples is through the REMS portal - they can delay or block access. A 2024 Health Affairs study found 78% of generic manufacturers reported REMS-related delays averaging 14.3 months before they could even start testing their product.
This isn’t just a paperwork problem. It’s a market problem. When generics can’t enter the market, prices stay high. Patients pay more. Insurance companies pay more. And the whole system pays more - even though the drug’s safety profile hasn’t changed.

What’s Changing? The Push for REMS Modernization
There’s growing recognition that REMS programs have become bloated, inconsistent, and outdated. The FDA launched its REMS Modernization Initiative in 2023 to fix that.
Here’s what’s coming:
- Standardized forms and digital portals instead of 10 different systems for 10 different drugs
- Electronic verification of prescriber certification - no more phone calls or faxes
- A new ‘REMS Dashboard’ launching in Q3 2025 to show how well each program is working
- A mandatory ‘REMS Assessment Standard’ by December 2025, required by the 21st Century Cures Act Reauthorization
Experts like Dr. Rachel Sherman, former FDA deputy commissioner, predict REMS will increasingly use real-world data - like electronic health records and pharmacy claims - to monitor safety instead of manual checklists. Imagine a system that flags a patient who missed three blood tests automatically, instead of waiting for a pharmacist to manually check a portal.
There’s also pressure to fix the generic drug access problem. The Health Affairs study recommends a 90-day legal window for generic companies to get samples. If the brand company doesn’t comply, they could face penalties.
Is REMS Worth It?
Yes - but only if it’s done right.
The FDA estimates REMS programs prevent $8.4 billion in healthcare costs each year by avoiding hospitalizations, transplants, and birth defects. That’s huge. But the $1.2 billion annual cost to the system isn’t just a number. It’s hours lost by doctors, delays for patients, and extra work for pharmacists.
REMS isn’t broken. It’s just outdated. It was built for a time when paper forms and phone calls were the norm. Now we have digital records, AI alerts, and real-time data. The goal shouldn’t be to remove REMS. It should be to make it smarter.
Patients still need access to life-saving drugs. Doctors still need protection from liability. Pharmacists still need clear rules. And the FDA still needs a way to ensure safety. The future of REMS isn’t about more paperwork. It’s about less friction - with the same level of protection.
What drugs require a REMS program?
Drugs with serious safety risks that can’t be managed through standard labeling alone require a REMS. Examples include lenalidomide (Revlimid) for multiple myeloma, clozapine for schizophrenia, thalidomide for leprosy and myeloma, isotretinoin for severe acne, and alemtuzumab (Lemtrada) for multiple sclerosis. These drugs carry risks like birth defects, severe infections, or life-threatening drops in blood cells. As of 2024, about 120 REMS programs cover 185 drugs - mostly in oncology, neurology, and autoimmune disease.
Who is responsible for running a REMS program?
The drug manufacturer is legally responsible for designing, funding, and managing the REMS program. They build the portals, train providers, distribute materials, and submit reports to the FDA. But the program only works if everyone else plays their part: doctors must get certified, pharmacists must verify requirements, patients must comply with monitoring, and specialty pharmacies must handle distribution. It’s a shared system - but the manufacturer carries the legal and financial burden.
Can a REMS program be removed?
Yes, but it’s rare. The FDA can remove a REMS if evidence shows it’s no longer needed to ensure the drug’s benefits outweigh its risks. Since 2007, only three REMS programs have been fully discontinued. The most recent was for Zeposia (ozanimod) in March 2023, after the FDA determined its safety risks were adequately managed through updated prescribing labels and routine monitoring. Removal requires formal review and public notice.
Why do pharmacists complain about REMS?
Pharmacists face time-consuming verification steps before dispensing REMS drugs. They must check online portals to confirm prescriber certification, patient enrollment, and required tests - often for multiple drugs with different systems. For drugs like Entyvio or Lemtrada, this can add 15-20 minutes per prescription. Many pharmacies don’t stock REMS drugs because of the complexity, forcing patients to use specialty pharmacies, which can delay treatment. The lack of standardized systems makes it harder and more error-prone.
How does REMS affect generic drug availability?
REMS can delay generic entry by blocking access to brand-name drug samples needed for testing. If the brand company controls the REMS portal and refuses to share samples, generic manufacturers can’t prove their product is equivalent. A 2024 study found 78% of generic companies faced delays averaging 14.3 months due to REMS-related access issues. This reduces competition, keeps prices high, and limits patient options - even when the drug’s safety profile is identical.
James Kerr
December 2, 2025 AT 13:57shalini vaishnav
December 3, 2025 AT 03:35vinoth kumar
December 4, 2025 AT 15:49bobby chandra
December 5, 2025 AT 14:32Archie singh
December 7, 2025 AT 08:46Gene Linetsky
December 8, 2025 AT 19:31Ignacio Pacheco
December 10, 2025 AT 01:16Jim Schultz
December 10, 2025 AT 22:57Kidar Saleh
December 11, 2025 AT 21:20Chloe Madison
December 13, 2025 AT 17:36Vincent Soldja
December 13, 2025 AT 23:19