Obesity isn’t just about eating too much or moving too little. It’s a medical condition rooted in broken biology - where the body’s natural systems for controlling hunger, fullness, and energy use stop working the way they should. This isn’t a failure of willpower. It’s a failure of signals. The brain, fat cells, gut, and pancreas are all talking to each other, but the messages are scrambled. And that’s why losing weight and keeping it off feels so hard for so many people.
The Brain’s Hunger Control Center
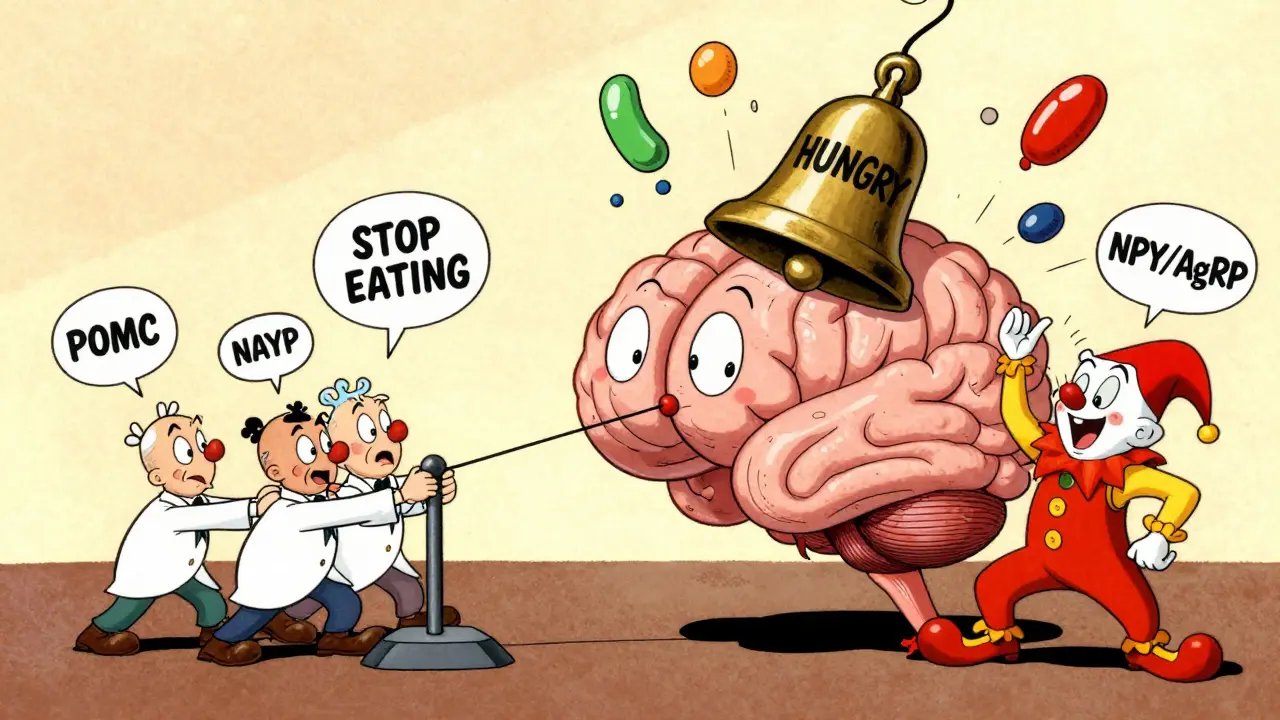
Deep in your brain, tucked inside the hypothalamus, is a tiny cluster of nerve cells called the arcuate nucleus. This is the command center for appetite. It doesn’t make decisions based on how hungry you feel - it responds to hormones from your body, like a thermostat adjusting temperature. Two opposing teams of neurons do the work: one tells you to stop eating, the other tells you to eat more.
The anorexigenic team - made up of POMC neurons - releases a chemical called alpha-MSH. This signal tells your brain, "You’re full." When these neurons fire, food intake drops by 25% to 40% in lab studies. Meanwhile, the orexigenic team - NPY and AgRP neurons - scream "HUNGRY!" Activate just these cells in a mouse, and it eats 300% to 500% more food in minutes. In humans, this system is always active. But in obesity, it gets stuck on "eat."
Hormones That Lie to Your Brain
Leptin is the fat cell’s way of saying, "I’ve got enough stored." It’s released in proportion to body fat - lean people have 5-15 ng/mL, while people with obesity often have 30-60 ng/mL. You’d think more leptin would mean less hunger. But in most obese people, the brain stops listening. This is called leptin resistance. It’s not that there’s not enough leptin - there’s too much, and the signal is ignored. Think of it like turning up the volume on a speaker that’s already blaring, but the listener can’t hear it anymore.
Insulin, the blood sugar hormone, also talks to the same brain region. In a healthy person, insulin rises after a meal and helps shut down hunger. But in obesity, insulin resistance in the brain means this signal weakens too. Ghrelin, the "hunger hormone," does the opposite. It spikes before meals - from 100-200 pg/mL to 800-1000 pg/mL - and directly activates the "eat" neurons. In people with obesity, ghrelin doesn’t drop as much after eating, so hunger lingers longer.

Why Your Brain Stops Listening
Leptin and insulin both use the same wiring in the brain: the PI3K/AKT pathway. This is the main channel for telling your brain to reduce food intake. When this pathway works, leptin cuts appetite by 30-50%. But in obesity, inflammation and excess fat trigger a brake on this system. One of those brakes is JNK, a stress-related enzyme that gets turned on in the hypothalamus. When JNK fires, it blocks leptin’s message. Another is PTEN, a protein that shuts down PI3K. Studies show mice with extra PTEN lose weight but still feel hungrier - proving that energy use and appetite are controlled by separate, overlapping circuits.
Even the mTOR system, which helps cells sense nutrients, gets disrupted. Stimulating mTOR in the brain reduces eating by 25% in obese mice. But in humans with long-term obesity, this system becomes sluggish. It’s like your car’s fuel gauge stops working - you keep driving even when the tank is empty.
Other Players in the Game
It’s not just leptin and insulin. Other hormones join the conversation - and sometimes, they make things worse.
- Pancreatic polypeptide (PP): Released after eating, it slows digestion and reduces hunger. But 60% of people with diet-induced obesity have PP levels too low - so their brain never gets the "I just ate" signal.
- Estrogen: After menopause, women gain weight around the waist. Why? Estrogen helps suppress appetite and boosts energy use. When estrogen drops, women eat 12-15% more and burn less energy. Mice without estrogen receptors eat 25% more and move 30% less.
- Serotonin: This mood chemical also affects hunger. Some studies say it works through the 5HT2C receptor to activate POMC neurons. Others say it’s the 5HT1B receptor that shuts down NPY neurons. The truth? Both might be right - and we’re still figuring out how.
- Orexin: This system keeps you awake and alert. In obesity, orexin levels drop by 40%, which might explain why people feel sluggish. But in night-eating syndrome, orexin spikes at night, driving late-night cravings.

How We’re Trying to Fix It
Scientists aren’t just studying the problem - they’re building solutions based on the biology.
Setmelanotide, a drug that activates the MC4R receptor (the same one triggered by alpha-MSH), helps people with rare genetic obesity lose 15-25% of their body weight. It works because it bypasses leptin resistance and speaks directly to the brain’s "stop eating" signal.
Semaglutide, originally for diabetes, mimics GLP-1 - a gut hormone that slows digestion and reduces appetite. In trials, it led to 15% average weight loss. It doesn’t just hit one pathway. It hits several: it reduces ghrelin, boosts PP, and increases insulin sensitivity.
The biggest breakthrough came in 2022: researchers found a new group of neurons next to the hunger and fullness cells. When they activated these neurons, eating stopped within 2 minutes. This wasn’t a hormone. It was a direct neural switch. It’s the first time we’ve seen a way to override hunger without touching leptin or insulin.
The Bigger Picture
Obesity affects 42.4% of U.S. adults. Globally, prevalence has nearly tripled since 1975. It’s not a lifestyle choice - it’s a disease of miscommunication. Your body is trying to tell you something. But the signals are corrupted by inflammation, fat accumulation, and decades of processed food.
What’s clear now is that treating obesity means fixing the biology, not just cutting calories. You can’t willpower your way out of leptin resistance. You can’t diet your way past a broken PI3K pathway. The future of treatment lies in drugs that restore these signals - or in combinations that hit multiple targets at once.
By 2030, WHO predicts 1 in 4 adults worldwide will have obesity. Without understanding how appetite and metabolism go wrong, we’ll keep treating symptoms instead of causes. And that’s why this science matters - not just for doctors, but for every person who’s ever been told to "just eat less."
Is obesity caused by eating too much?
Not directly. While overeating contributes, the root cause is biological: the body’s hunger and fullness signals get disrupted. In most cases, people with obesity aren’t eating more because they lack discipline - they’re eating more because their brain isn’t receiving or responding to the hormones that say "stop." Leptin resistance, insulin resistance, and low PP levels all trick the brain into thinking it’s starving, even when fat stores are full.
Why don’t weight loss drugs work for everyone?
Because obesity isn’t one disease - it’s many. Some people have strong leptin resistance, others have low PP or disrupted serotonin signaling. Drugs like semaglutide work best when they match the underlying biology. A drug targeting the MC4R receptor won’t help someone whose main issue is low orexin. That’s why future treatments will need to be personalized, based on hormone profiles and genetic factors, not just BMI.
Can you reverse leptin resistance?
Yes - but not easily. Studies show that significant weight loss (10% or more of body weight) can improve leptin sensitivity. However, the brain often resets its "set point," so hunger returns even after weight loss. The best approach combines sustained lifestyle changes with medications that restore signaling. There’s no quick fix, but long-term metabolic health can improve with consistent, targeted intervention.
Do hormones explain why women gain weight after menopause?
Yes. Estrogen helps regulate appetite and energy use. When estrogen drops after menopause, the brain’s hunger signals become more active, and fat-burning drops by 15-20%. Studies in mice show that removing estrogen receptors leads to 25% more eating and 30% less movement. This isn’t just about fat distribution - it’s a direct effect on the brain’s appetite control centers.
Is there a genetic component to obesity?
Absolutely. Mutations in genes like LEPR, POMC, and MC4R can cause severe obesity from childhood. These are rare - fewer than 50 cases worldwide - but they prove that appetite regulation is genetically wired. Even common obesity has strong genetic links: over 1,000 gene variants have been tied to BMI. Genetics don’t determine your fate, but they shape how your body responds to food, stress, and sleep.
Why do people regain weight after losing it?
Your body fights to return to its "set point." After weight loss, leptin drops, ghrelin rises, and metabolism slows. The brain interprets this as starvation and ramps up hunger. This isn’t laziness - it’s survival biology. That’s why long-term weight maintenance often requires ongoing support, whether through medication, behavioral therapy, or both. The body isn’t broken - it’s trying to protect you.
Can diet alone fix metabolic dysfunction?
Diet alone rarely fixes the underlying biology. Restricting calories without addressing insulin resistance, inflammation, or hormone signaling often leads to short-term loss and long-term regain. The most effective approach combines nutrition with therapies that restore signaling - like GLP-1 agonists or future drugs targeting the newly discovered neurons. Food is part of the solution, but not the whole one.
What’s the most promising new treatment for obesity?
The most exciting development is the discovery of excitatory neurons next to the appetite centers that can shut down eating within 2 minutes. Unlike drugs that mimic hormones, this targets the brain’s wiring directly. Early animal studies show it’s possible to turn off hunger without side effects. Human trials are expected to start soon. If successful, this could lead to the first non-hormonal, brain-specific obesity treatment.
Mike Hammer
February 14, 2026 AT 15:13Also, the part about orexin dropping in obesity? That explains why I feel like a zombie after lunch. Not laziness. Just my brain got lazy.
Sarah Barrett
February 14, 2026 AT 15:43It's time we reframe this as a neurological condition - not a character flaw.